Status of HIFU in the US
As of October 13, 2015, SonaCare Medical Receives FDA Clearance for its Sonablate® High Intensity Focused
Ultrasound Prostate Ablation Device. The Device will start distribution in October.
The authorization of use of the Sonablate® in the U.S. is a milestone for non-invasive prostate care and a tremendous gain for men's health.
Formerly, the device was only available for use in Canada, Mexico, Bermuda, the Bahamas, The Dominican Republic, Argentina and Europe.
Contact Riverside Urology today at
614-442-3000 to learn more about this revolutionary, minimally invasive treatment now available as a treatment option in the U.S.
Brief History of HIFU
HIFU research began in the 1950s in Indianapolis, IN.
In 2004, the first International HIFU Centers opened in the Americas, outside of the United States.
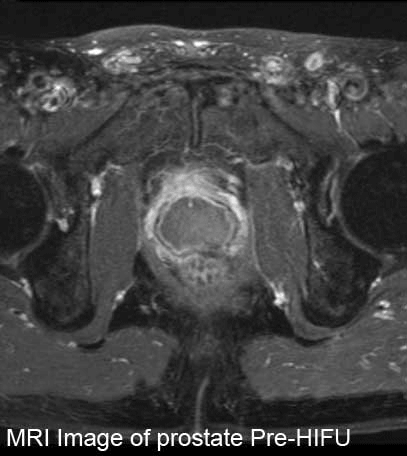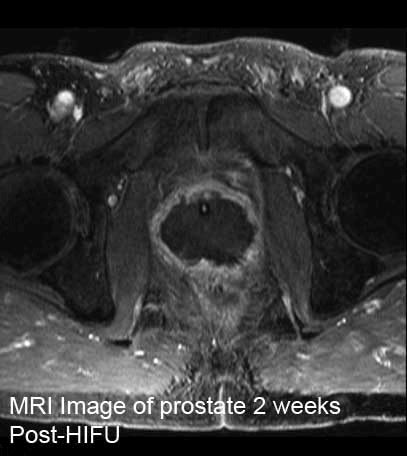
MRI Image of prostate Pre-HIFU vs Post-HIFU
These are T1.5 Tesla MRI's. The first MRI shows a patient prior to the HIFU procedure.
The second image shows the prostate 2 weeks Post-HIFU. The entire "meat" of the prostate is gone.
The patient is able to urinate with
good control and has retained sexual function. He is a relatively young person.